Regulatory considerations for digital surrogate endpoint response biomarkers
On July 24, 2023, I gave a “quick” talk on regulatory considerations at the EverythingALS Digital Biomarkers Summit. This post is adapted from that talk.
Regulatory Considerations for Digital Biomarkers

My name is Brendan O’Leary, and, up until a few months ago, I was a federal regulator. I spent 14 years with the FDA’s Center for Devices and Radiological Health, or CDRH. For about 10 of those years, I worked in the diagnostics office, where my focus was on radiology devices, software, and digital health. Then, I helped launch the FDA’s Digital Health Center of Excellence as its founding deputy director, and I led the Center of Excellence throughout 2022.
My education is in engineering, and, over the years, I helped the FDA make somewhere on the order of a thousand decisions related to digital health, biomarkers, and diagnostics, including decisions on companion diagnostics, complementary diagnostics, and digital biomarkers used with drugs. I’ll approach this topic from that experience today.
I’ll start by covering definitions for terms like “digital biomarker,” “response biomarker,” and “surrogate endpoint response biomarker.”
From there, I’ll discuss the differences between a biomarker and a clinical outcome assessment, including clinical outcome assessments enabled by digital health technologies.
Then, I’ll cover what a useful biomarker or other measure should do.
And finally, I’ll end with a few thoughts about how to convince the clinical community, payors, and the FDA that your biomarker or other measure is actually useful.
Biomarker: The “Best” Definition

The most important thing to know about digital biomarkers is that they are biomarkers. “Digital” is not the most important part. “Digital” is a reference to enabling technology. It’s a description of how we measure a biomarker or how we transmit information about a biomarker. That enabling technology is often critical: Sometimes, it’s the only reason it’s possible to measure or use the biomarker at all! But it’s still a means to an end, and it’s the end that’s most important here: From a regulatory perspective, the most important thing about digital biomarkers is that they are biomarkers.
So, let’s focus on that for now: What is a biomarker?
Back in 2016, some folks from the FDA’s device and drug centers and from the NIH got together to define this and other terms. They published the cleverly-but-perhaps-not-so-humbly-named “BEST Resource,” [1] which you can find at the link in the QR code on this slide. The document is about 60 pages, and it’s an important read if you ever want to communicate clearly with the FDA on this topic.
Among other things, the BEST Resource defines the terms biomarker and digital health technology.
And, based on this, according to the FDA, a biomarker, including a digital biomarker, is a characteristic measured as an indicator of a biological process, whether that process is normal or affected by a disease.
Equally important is what a biomarker is not. A biomarker is not a measure of how someone feels, functions, or survives. We often call measures of how someone feels, functions, or survives “outcomes,” and I’ll say more about these in a few moments. But the thing to keep in mind is that a biomarker is not itself an outcome.
The BEST Resource lays out several categories of biomarkers. There are two important things to know about these categories:
First, the categories are defined based on their context of use – which is very similar to “intended use” if you’re familiar with that term in regulatory contexts – and,
Second, the categories are not mutually exclusive: a biomarker can be both a prognostic biomarker and a predictive biomarker, for example.
On that first point, when I say that the biomarker categories are defined based on the context of use of the biomarker, that means that the categories are more about how a biomarker is used than about what a biomarker is measuring. That’s why the same biomarker can belong in more than one category depending on how it is being used. For example, hemoglobin A1c (HbA1c) can be used as a diagnostic biomarker to identify patients with Type 2 diabetes. It can be measured repeatedly over time as a monitoring biomarker to assess disease progression. And, it can be used as a response biomarker to evaluate diabetes control following treatment with a drug. You are measuring the same thing in each case, but it is how you are using the information that drives what words you should use to describe the biomarker.
These different contexts of use for a biomarker are all relevant in medical product development, but today, we’ll focus on the use of biomarkers as response biomarkers in particular. This use has well-defined regulatory considerations laid out in the BEST Resource.
Response Biomarker
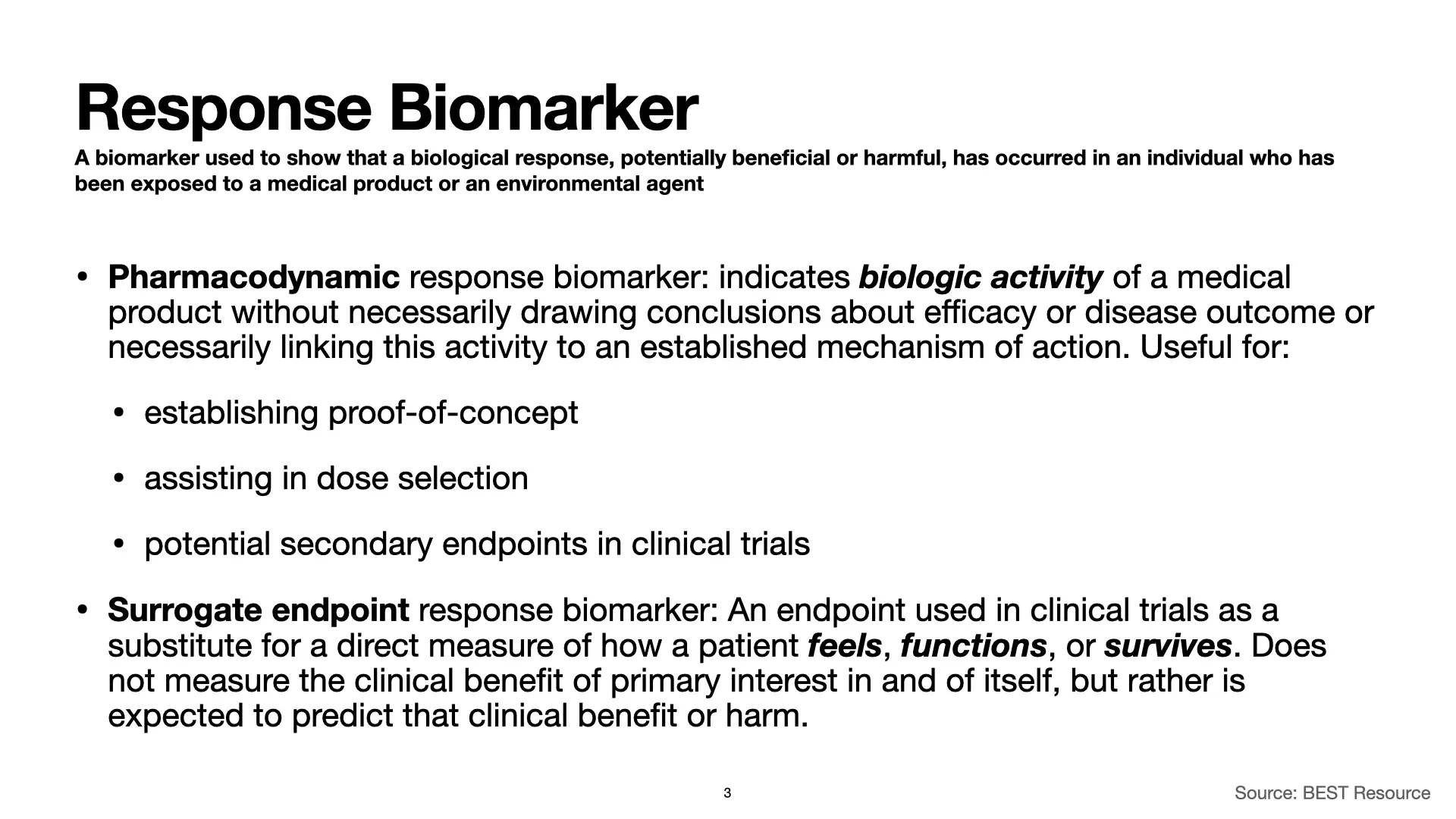
When we use a biomarker as a response biomarker, we’re using it to measure a response. Typically, we’re measuring a response to a drug or other medical product. If we’re only looking for evidence of biological activity and are not trying to use the biomarker to assess a disease outcome, we’re probably using the biomarker as a pharmacodynamic response biomarker. In contrast, when we use a biomarker as a measure of whether we can expect that a therapeutic is providing a clinical benefit, like whether a therapeutic is likely to have a positive impact on how a patient feels, functions, or survives, we’re using the biomarker as a surrogate endpoint response biomarker. Surrogate endpoint response biomarkers are not direct measures of the expected clinical benefit itself. They are measures that we hope or expect to correlate with that benefit.
Surrogate Endpoint Response Biomarkers
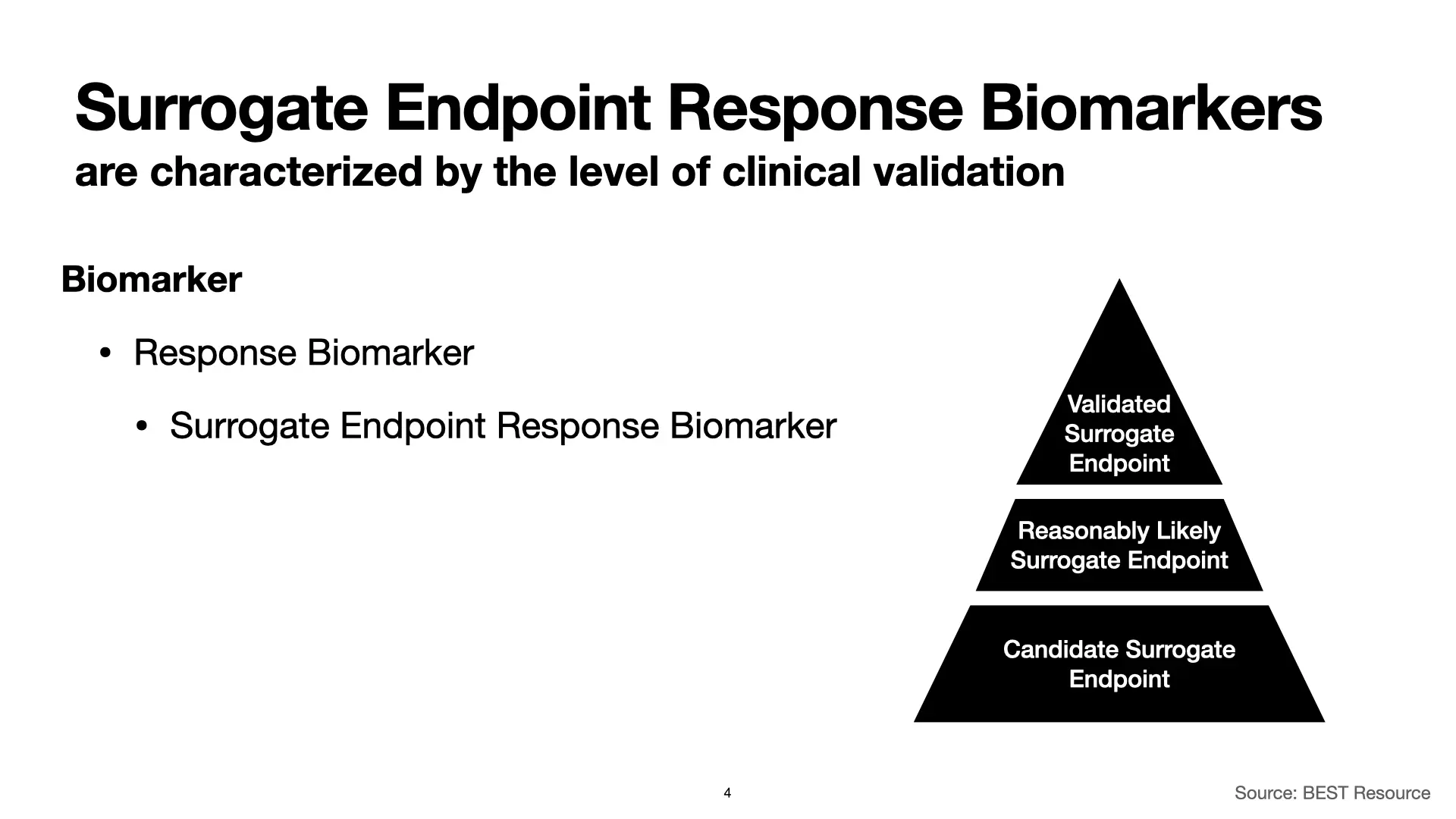
We’ve covered biomarkers and digital biomarkers. Within that category, we’ve covered a number of types of biomarkers and have focused on response biomarkers in particular. From there, we zoomed in on surrogate endpoint response biomarkers. And now, within that sub-sub-category, we’ll cover three different types of surrogate endpoint response biomarkers.
Surrogate endpoint response biomarkers are characterized by the level of clinical validation. At the top, with the most evidence, we have “Validated Surrogate Endpoints.” Moving down from there, we have “Reasonably Likely Surrogate Endpoints” and “Candidate Surrogate Endpoints.” Each is associated with different levels of evidence for their validity, and that has implications for using them in clinical studies to support FDA authorizations of therapeutic products.
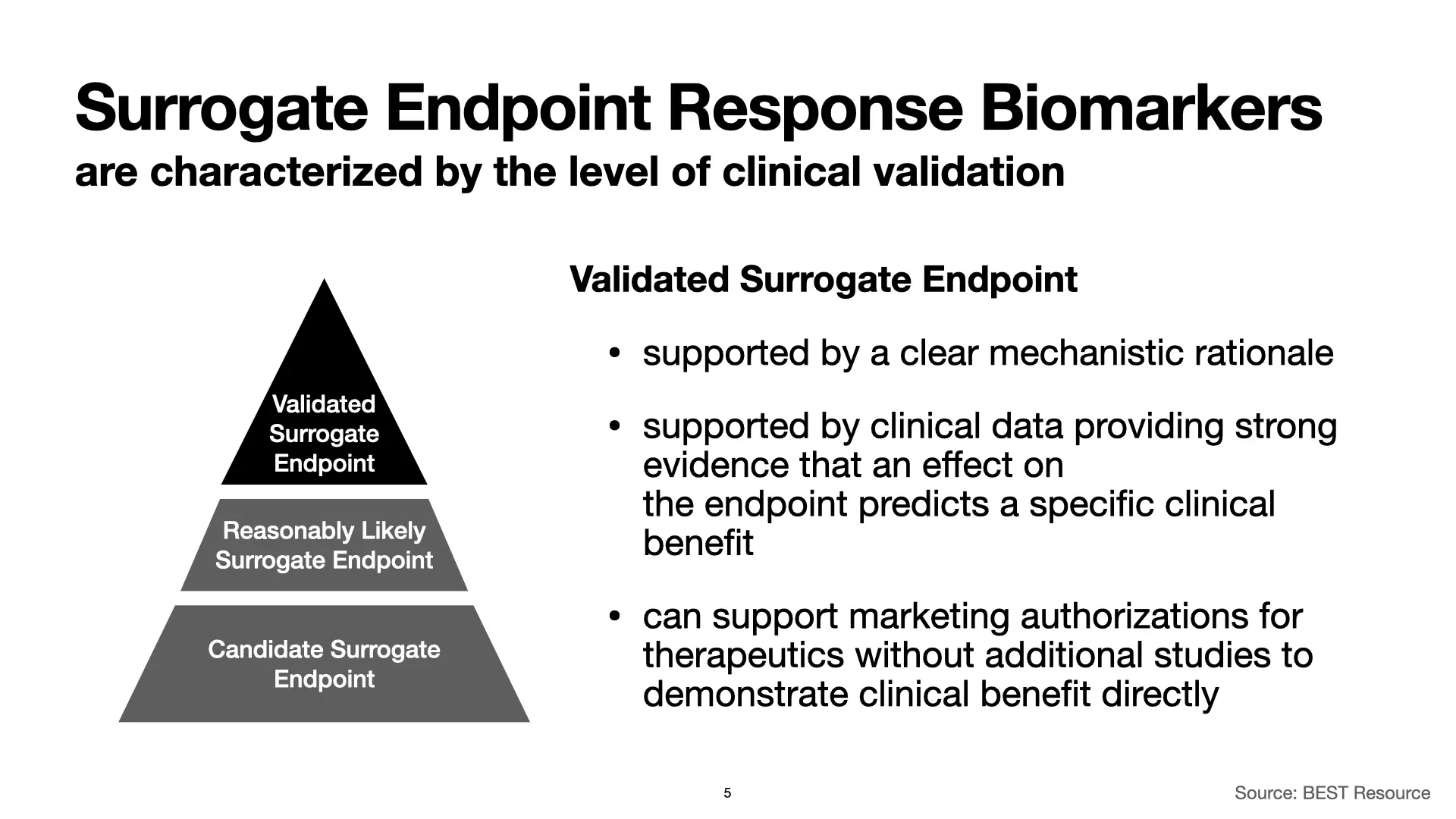
Let’s start with validated surrogate endpoints. The BEST Resource identifies two characteristics of these surrogate endpoints.
First, we have a clear mechanistic rationale for validated surrogate endpoint response biomarkers. That means that we understand the link between the biomarker and the mechanisms of the disease that we’re studying. The link is known, and it’s clear.
Second, clinical data shows that an effect on a validated surrogate endpoint response biomarker predicts a clinical benefit, and we know from the clinical data what that clinical benefit will be.
If you have these two things, you have everything you need. A validated surrogate endpoint response biomarker can support FDA marketing authorizations for therapeutics without additional studies. Seeing an effect on a validated surrogate endpoint response biomarker is enough, and if you have that, you don’t need additional pre- or postmarket studies that directly demonstrate that the therapeutic has a clinical benefit.
This is the mountaintop. This is what we want to attain.
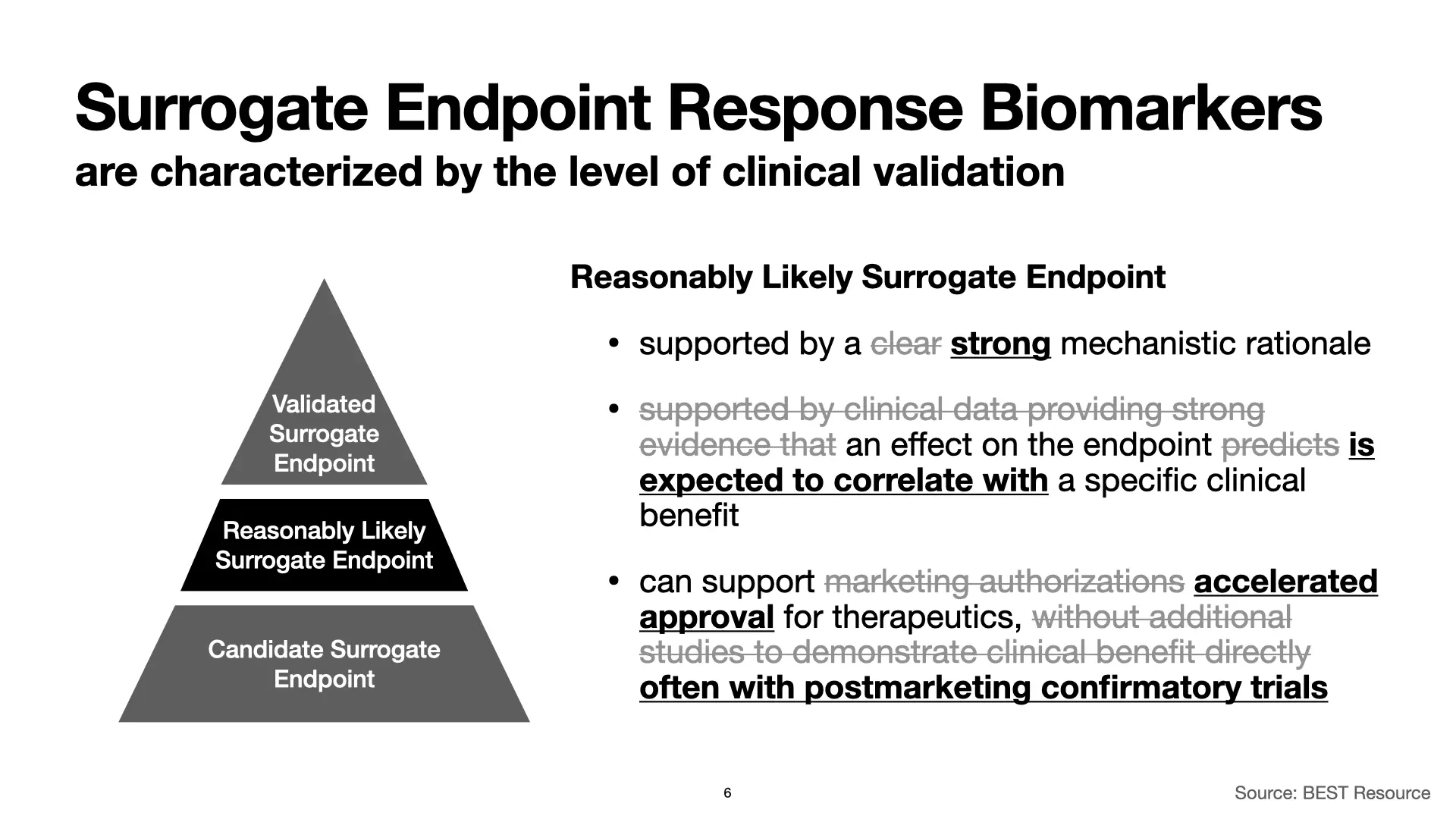
It’s a long journey to get to a validated surrogate endpoint response biomarker, and on the way there, you can find yourself with some really good indications that you have a good biomarker on your hands, but without all of the evidence you would need to say that it’s validated. This is called a “reasonably likely surrogate endpoint response biomarker,” and it’s easiest to introduce these by highlighting how they differ from validated surrogate endpoint response biomarkers.
First, while for a validated surrogate endpoint response biomarker we have a clear understanding of the link between the biomarker and the mechanisms of the disease, for a reasonably likely surrogate endpoint response biomarker, we only have a strong rationale: We have a really good, really plausible theory, but not enough to convince a regulator.
Second, instead of having clinical data that shows that an effect on the surrogate endpoint response biomarker predicts a specific clinical benefit, for reasonably likely surrogate endpoint response biomarkers, we only have an expectation that the biomarker will correlate with a specific clinical benefit. Notably though, based on the strong mechanistic rationale, we should still have a specific expectation for how a specific clinical benefit will correlate with the biomarker.
As a result of the increased uncertainty associated with reasonably likely surrogate endpoint response biomarkers, they alone can’t support full FDA marketing authorizations for therapeutics without additional studies. But they are still incredibly powerful, and they can support accelerated approval of therapeutics, often with postmarketing confirmatory trials.
And, if you plan things right, and if the science works out the way you hope it will, those confirmatory trials can validate the surrogate endpoint response biomarker at the same time as they validate the therapeutic.
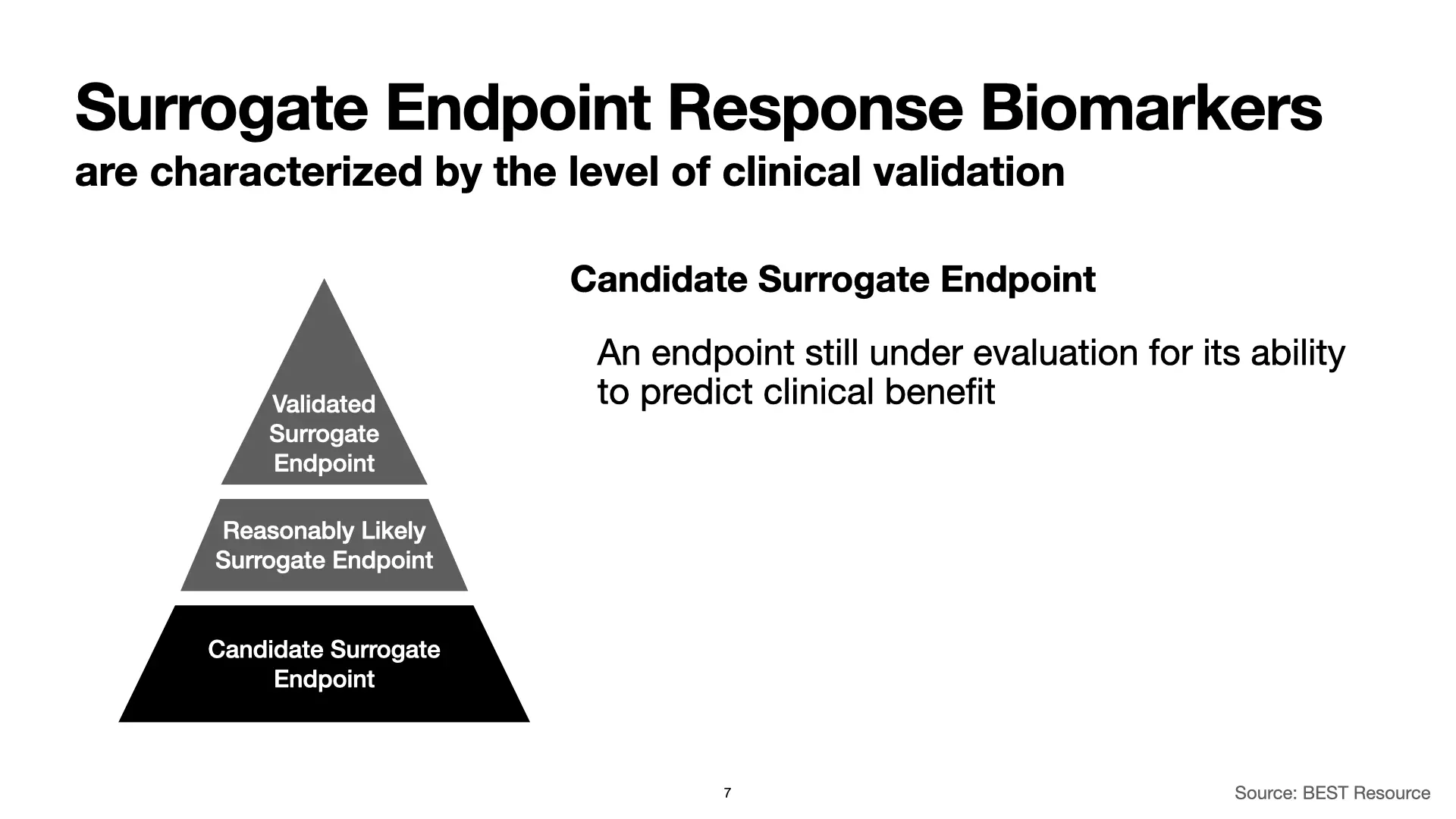
Finally, we have candidate surrogate endpoint response biomarkers. These are all of the things that we’re working to develop and hope will work, but for which we don’t yet have the data.
Clinical Outcome Assessment (COA)
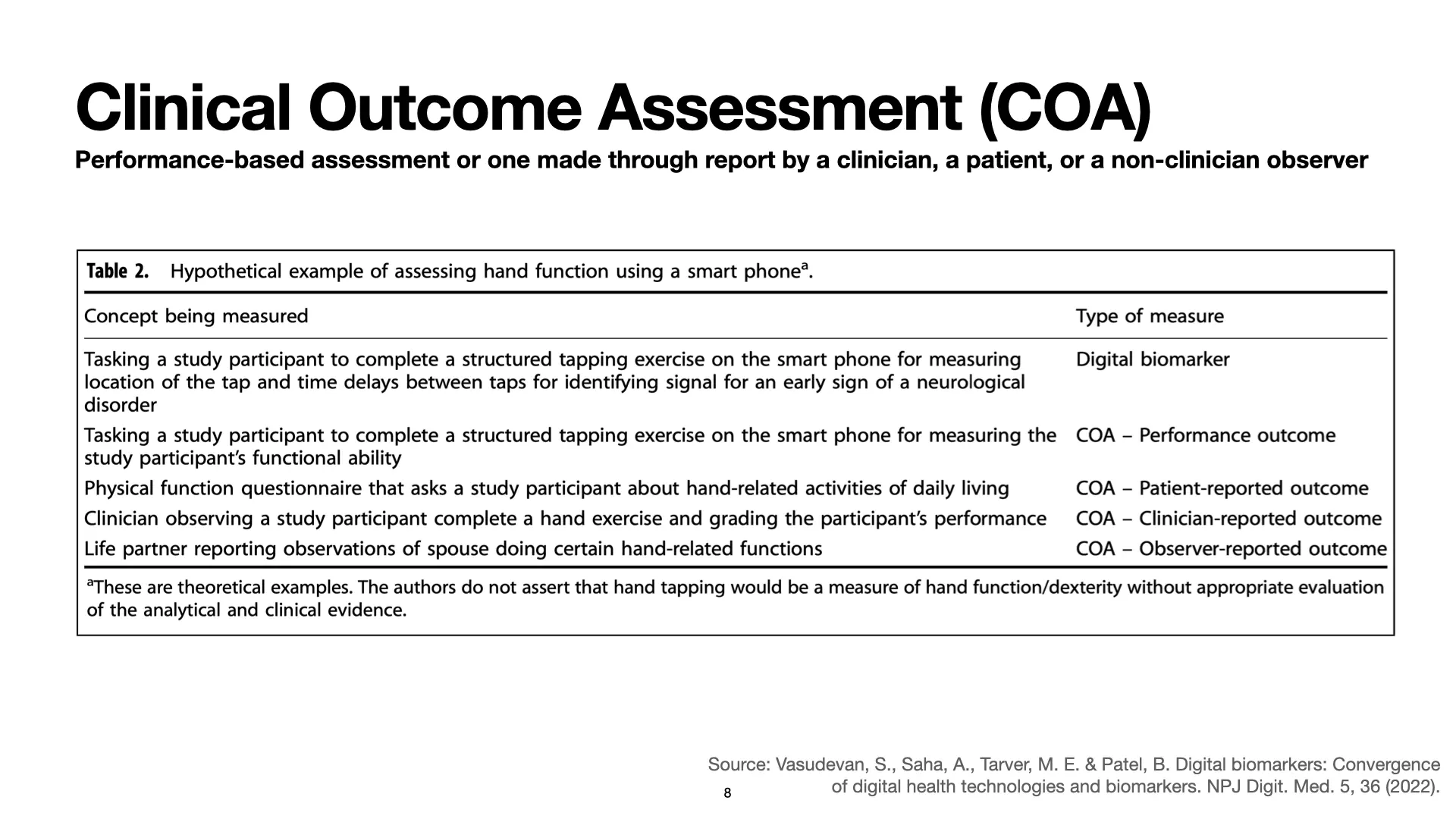
At the beginning of this post, I said that part of understanding what a biomarker is involves understanding what it is not. A biomarker is an indicator of a biological process. A biomarker is not a measure of how someone feels, functions, or survives. How someone feels, functions, or survives is an outcome, and one way to measure an outcome is to use a Clinical Outcome Assessment, or COA.
Clinical Outcome Assessments are performance-based assessments or assessments made by a clinician, a patient, or another observer. Depending on who is doing the assessment, a COA may be a patient-reported outcome (PRO), a clinician-reported outcome, or an observer-reported outcome. Sometimes, like with biomarkers, COAs may be enabled or facilitated by digital technologies. When people want to emphasize this, they sometimes stick the word “electronic” to the front of the term, and that’s where you get acronyms like “ePRO.” It just keeps going: There is no shortage of more and more specific jargon and acronyms for all of these concepts.
But, for our purposes, just know that an outcome is not a biomarker, and so a digitally facilitated outcome assessment is not a digital biomarker. Some of my colleagues at the FDA put together a nice paper describing this and showing how, based on the context of use and the concept being measured, very similar measurement technologies can fall into many different categories. [2]
Step 1: Find something meaningful

Find something meaningful to measure.
Identify the gaps between the measures we have today and the needs of patients, trial participants, clinicians, and therapeutic product developers. Find ways to fill those gaps.
Peter Fernandes put it well here. [3] He was talking about what he was hearing from people with pulmonary fibrosis. And the bottom line is, our traditional measures aren’t cutting it. We’ve absolutely got to do better.
There are legacy measures that have been used for a long time, like the six-minute walk test in this example or the ALS-FRS. But Fernandes points out the shortcomings of those measures from a patient perspective. And, to be blunt, those measures are a hot mess from a scientific and regulatory perspective as well. There is just so much noise in them. Any researcher who has used them will tell you their limitations, which are numerous and extensive. But we still use them.
And, as awful as these measures are, we have good reason to keep using them: In part, it’s because decades of experience have given us such a good sense for the particular limitations of these measures. We know a lot about their strengths and their weaknesses.
So, as you look for something new and more meaningful to measure, keep in mind that there is a lot to be said for planning to measure it alongside the measures that we’re already familiar with, at least for a while. Doing that is part of the process of confirming that the new measures we’re crafting really are meaningful and really are better.
Which brings us to Step 2.
Step 2: Measure it

Measure it.
This is the part of the post where I make the “big reveal” and tell you that you don’t really need to know that much about digital biomarkers, clinical outcome assessments, or any of the rest of this. The bottom line is that they are all things you measure or measurements. So, let’s ignore the BEST Resource and all of its biomedical jargon and definitions and just focus on what a measurement is.
This definition comes from the field of information theory and is in a book by Douglas Hubbard. [4] The definition identifies three key features of a measurement:
First, a measurement is a reduction of uncertainty. This isn’t about knowing “for sure.” Few if any of our existing measures get us there, and our new measures don’t need to get us to certainty either. They just need to meaningfully reduce our uncertainty.
Second, a measurement is based on one or more observations. I don’t have much to say about this. It’s what science is all about. It’s empiricism in a nutshell.
Finally, with a measurement, we can quantitatively express the reduction in uncertainty that these observations provide. For example, both qualitative and quantitative measurements may produce confidence intervals that can be expressed quantitatively. Regulators love confidence intervals, and regulators especially love to see a confidence interval shrink.

So that’s the real message. Digital biomarkers are exciting. Digital Health Technologies that enable new Clinical Outcome Assessments are too. It’s all good stuff. But don’t get too caught up in all of that. At the end of the day, the real key here is to measure something meaningful.
If you can find something meaningful, if you can reduce the uncertainty around that meaningful something, and if you can express that reduction quantitatively, you’ve made progress.
And if you can do all of that, then you’ve got a good case to make to the FDA and others.
References
Reuse
Citation
@online{o'leary2023,
author = {O’Leary, Brendan},
title = {Regulatory Considerations for Digital Surrogate Endpoint
Response Biomarkers},
date = {2023-11-14},
url = {https://www.boleary.com/blog/posts/202311-digital-biomarkers/},
langid = {en}
}

